Estri Gateway Health Canada, Xml Workshop Xml Standardformat Fur Den Austausch Von Elektronischen Daten In Der Pharmazeutischen Industrie Joerg Dillert Senior Consultant March Ppt Herunterladen
It allows submission of electronic applications to both Health Canada and the US. Gateway Traders Prerequisites for a Gateway Trader.
Https Admin Ich Org Sites Default Files Inline Files 11 Pharmacovigilance Challanges Pdf
A regulatory activity in Non-eCTD Hybrid format is a full electronic regulatory activity that is accompanied by Modules 1 and 2 in paper-based format.

Estri gateway health canada. For example a company may want to send ISCR reports via the Gateway-to-Gateway exchange and other submissions via the FDA ESG web interface. DynaLIFEDXWalk in service and appointment booking accepted. 780-702-4486 Appointment Line 780-702-0551 Lab.
ESTRI Gateway but those that participated in the pilot. Companies wishing to use the Gateway need to have access to the Electronic Standards for the Transfer of Regulatory Information ESTRI Gateway but those that participated in the pilot can continue to submit applications without having to register again. E2B MedDRA ESTRI-Gateway Management ICSR reporting Scanningggg and Imaging Capability for future integration document management solution.
Core Application For the collection coding assessment and reporting of adverse reaction data Workflow module routes AR cases to SpecialistsAssessors. Electronic Standards for the Transfer of Regulatory Information ESTRI-Gateway 10. Health Canada does not establish or fund registries.
The reporting of ARs to Health Canada Domestic AR report information is entered into the CanadaDomestic AR report information is entered into the Canada. With the passage of the Food Drug and Cosmetic Act in 1938 the US. In January 2013 Health Canada and the FDA entered into a Cooperative Research and Development Agreement CRADA.
At this time GoSecure does not support your browser. In March 2010 Health Canada began the Non-eCTD Hybrid Pilot as a transitory aid for sponsors not yet ready to submit in eCTD format. The information within this presentation is based on the presenters expertise and experience and represents the views of the presenter for the purposes of a training workshop.
ESTRI gateway module 22. Public health is the primary mission of the FDA the mechanism by which the agency accomplishes this task has undergone several organizational transformations. Regular Pharmacovigilance TrainingsSeminars 11.
Toutefois vous serez peut-être malgré tout capable daccéder aux services utilisant GoSecure. Learn the latest GIS technology through free live training seminars self-paced courses or classes taught by Esri experts. Application users consist of World Health Organization WHO Physicians Pharmacists and the public.
When a pharmaceutical company receives ICSRs through ESTRI gateway the reported productsactive ingredients are expected to map either with the productsingredients maintained in the CPD maintained within ARISg or with productssubstances in the WHO Drug Dictionary. The CRADA agreement authorizes the Health Products Food Branch HPFB and the FDA to collaborate on a joint initiative to deliver an Industry-facing electronic Submissions gateway beginning with the adaptation and sharing of the FDAs Electronic Submissions Gateway for regulatory. Food and Drug Administration for pharmaceutical and biological products.
À lheure actuelle GoSecure ne prend pas officiellement en charge votre navigateur. Resources are available for professionals educators and students. Government first required products to.
Subject matter expertise in validating transition to eICSR XML submissions through ESTRI Gateway and. Health Canada February 05 2009 Disclaimer. This public meeting will provide insight on key topics that may reach the forefront in efforts of harmonization among regulatory bodies in the United States Canada Europe and Japan.
34 Canada Vigilance Database. The scenario described above is acceptable. Food and Drug Administration FDA including registrations listings and other notifications.
Encourage and facilitate information exchange among the NRAs on national laws regulations and establish guidelines and policies relating to the regulatory control of domestic or imported vaccines 2. Can create E2BR2R3 XML ICSRs from their pharmacovigilance database Have an ESTRI compliant automated gateway software solution for exchanging ICSRs and acknowledgments with the EudraVigilance Gateway Gateway Traders. FDA Industry Systems FIS was created to facilitate making submissions to the US.
Regulatory intelligence services in support of eReporting drug safety surveillance and audit preparedness as well as pharmacovigilance system consultations for quality measurement planning and implementation. However you may still be able to access GoSecure enabled services. While protecting the US.
The purpose of the project was to upgrade the existing web based infrastructure software and hardware associated with the ARISg ESTRI Gateway and agSignals applications supporting Health Canadas Canada Vigilance Program.
Http Publications Gc Ca Collections Collection 2021 Sc Hc H164 293 2019 Eng Pdf
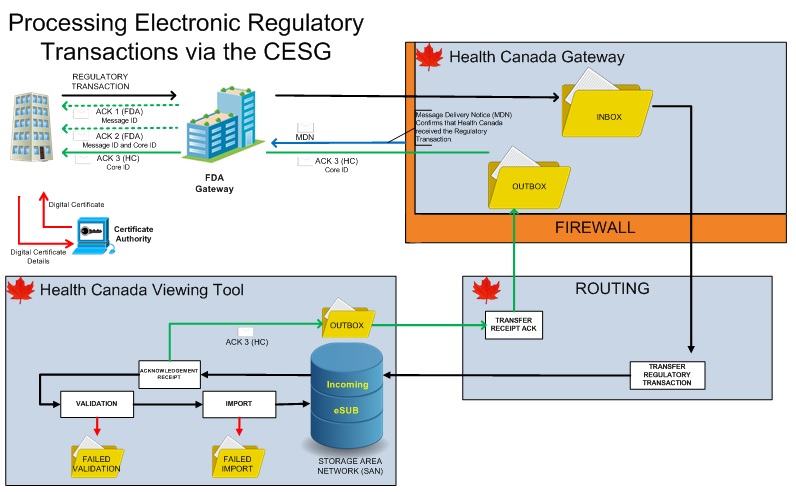
Frequently Asked Questions Common Electronic Submissions Gateway Canada Ca

Frequently Asked Questions Common Electronic Submissions Gateway Canada Ca

Xml Workshop Xml Standardformat Fur Den Austausch Von Elektronischen Daten In Der Pharmazeutischen Industrie Joerg Dillert Senior Consultant March Ppt Herunterladen
Pharmacovigilance Of Herbal Medicines Pharmacovigilance Phases Of Clinical Research

New Installation Of S 4hana 1809fps0 Part 3 Best Practices Content Activation Sap Blogs

Canada Vigilance Adverse Reaction Monitoring Program And Database Pdf Free Download
Https Www Fdanews Com Ext Resources Files 07 07 14 Hcegateway Pdf

Health Product Infowatch January 2020 Canada Ca
Https Www Ema Europa Eu Documents Other Icmra Mapping Pharmacovigilance Initiatives En Pdf

Frequently Asked Questions Common Electronic Submissions Gateway Canada Ca

Eudravigilance System Overview European Medicines Agency
Https Www Hemophilia Ca Files Heather 20sutcliffe 20 20medeffecttm 20canada 20 20canada 20vigilance 20program 20and 20database Pdf

Health Canada Issues A New Guidance Gui 0064 Spharm Canada S Drug Regulatory Experts
Http Publications Gc Ca Collections Collection 2021 Sc Hc H164 293 2019 Eng Pdf
Https Www Dgra De Media Pdf Studium Masterthesis Master Da Silva Lais 2018 Pdf

Health Canada Module 1 Updates And Progess Towards Topra

Frequently Asked Questions Common Electronic Submissions Gateway Canada Ca

Xml Workshop Xml Standardformat Fur Den Austausch Von Elektronischen Daten In Der Pharmazeutischen Industrie Joerg Dillert Senior Consultant March Ppt Herunterladen
